CBSE Class 12 Chemistry – MCQ and Online Tests – Unit 13 – Amines
Every year CBSE conducts board exams for 12th standard. These exams are very competitive to all the students. So our website provides online tests for all the 12th subjects. These tests are also very effective and useful for those who preparing for competitive exams like NEET, JEE, CA etc. It can boost their preparation level and confidence level by attempting these chapter wise online tests.
These online tests are based on latest CBSE Class 12 syllabus. While attempting these our students can identify the weak lessons and continuously practice those lessons for attaining high marks. It also helps to revise the NCERT textbooks thoroughly.
CBSE Class 12 Chemistry – MCQ and Online Tests – Unit 13 – Amines
Question 1.
The most convenient method to prepare primary (i Amine) amine containing one carbon atom less is
(a) Gabriel phthalmidie synthesis
(b) Reductive amination of aldehydes
(c) Hofmann bromamide reaction
(d) Reduction of isonitriles
Answer
Answer: (c) Hofmann bromamide reaction
Question 2.
Amides may be converted into amines by a reaction named after
(a) Hofmann Bromide
(b) Claisen
(c) Perkin
(d) Kekule
Answer
Answer: (a) Hofmann Bromide
Question 3.![]()
in the above reaction is
(a) CH3CH2CH2NHCOCH3
(b) CH3CH2CH2NH2
(c) CH3CH2CH2CONHCH3
(d) CH3CH2CH2CONHCOCH3
Answer
Answer: (a) CH3CH2CH2NHCOCH3
Question 4.
Identify the correct pathway to convert propanoic acid to ethylamine. The reagent represented by A, B and C are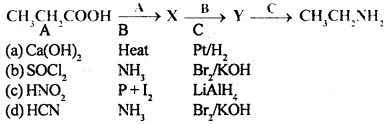
Answer
Answer: (b)
Question 5.
When excess of ethyl iodide is treated with ammonia, the product is
(a) ethylamine
(b) diethylamine
(c) triethylamine
(d) tetrathylammonium iodide
Answer
Answer: (d) tetrathylammonium iodide
Question 6.
Reduction of CH3CH2NC with hydrogen in presence of Ni or Pt as catalvst gives
(a) CH3CH2NH2
(b) CH3CH2NHCH3
(c) CH3CH2NHCH2CH3
(d) (CH3)3N
Answer
Answer: (b) CH3CH2NHCH3
Question 7.
Which of the following amides will give ethylamine on reaction with sodium hypobromide?
(a) Butanamide
(b) Propanamide
(c) Acetamide
(d)Benzamide
Answer
Answer: (b) Propanamide
Question 8.
Nitrogen atom of amino group is ………. hybridised.
(a) sp
(b) sp2
(c) sp3
(d) sp3d
Answer
Answer: (c) sp3
Question 9.
Which one of the following reducing agents is likely to be most effective in bringing about the following change?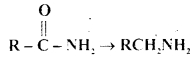
(a) H2-Ni
(b) NaBH4
(c) LiAlH4 ether
(d) Na-AIcohol
Answer
Answer: (c) LiAlH4 ether
Question 10.
Amine that cannot be prepared by Gabricl-Phthalmidie synthesis is
(a) aniline
(b) benzyl amine
(c) methyl amine
(d) iso-butylamine
Answer
Answer: (a) aniline
Question 11.
Arrange the following compounds in increasing order of basicity:
CH3NH2, (CH3)2 NH, NH3, C6H5NH2
(a) C6H5NH2 < NH3 < (CH3)2NH < CH3NH2
(b) CH3NH2 < (CH3)2NH < NH3 < C6H5NH2
(c) C6H5NH2 <NH3 < CH3NH2<(CH3)2NH
(d) (CH3)2NH < CH3NH2 <NH3 < C6H5NH2
Answer
Answer: (c) C6H5NH2 <NH3 < CH3NH2<(CH3)2NH
Question 12.
Which of the following is used as Hinsberg’s reagent?
(a) C6H5SO2Cl
(b) C6H5SO3H
(c) C6H5NHCH3
(d) C6H5COCH3
Answer
Answer: (a) C6H5SO2Cl
Question 13.
What is the end product in the following sequence of reactions?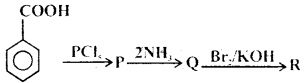
(a) Aniline
(b) Phenol
(c) Benzene
(d) Benzenediazxonium chloride
Answer
Answer: (a) Aniline
Question 14.
Secondary amines can be prepared by
(a) reduction of nitro compounds
(b) oxidation of N-substituted amides
(c) reduction of isonitriles
(d) reduction of nitriles
Answer
Answer: (c) reduction of isonitriles
Question 15.
Which of the following amines will give carbylatnine reaction?
(a) (C2H5)3N
(b) (C2H5)2NH
(c) C2H5NH2
(d) C3H7NHC2H35
Answer
Answer: (c) C2H5NH2
Question 16.
Benzoic acid is treated with SOCl2 and the product (X) formed is reacted with ammonia to give (Y). (Y) on reaction with Br2 and KOH gives (Z). (Z) in the reaction is
(a) aniline
(b) chlorobenzene
(c) benzamide
(d) benzoyl chloride
Answer
Answer: (a) aniline
Question 17.
The end product Z of the reaction![]()
(a) propanenitrile
(b) triethylamine
(c) diethylamine
(d) propylamine
Answer
Answer: (a) propanenitrile
Question 18.
Tertiary amines have lowest boiling points amongst isomeric amines because
(a) they have highest molecular mass
(b) they do not form hydrogen bonds
(c) they are more polar in nature
(d) they are most basic in nature
Answer
Answer: (b) they do not form hydrogen bonds
Question 19.
Primary and secondary amines are distinguished by
(a) Br2/ROH
(b) HClO
(c) HNO2
(d) NH3
Answer
Answer: (c) HNO2
Question 20.
Which of the following species are involved in the carbvlamine test?
(i) RNC
(ii) CHCl3
(iii) COCl2
(iv) NaNO2 + HCl
(a) (i) and (iv)
(b) (i) and (ii)
(c) (ii) and (iv)
(d) (ii) and (iii)
Answer
Answer: (b) (i) and (ii)
Question 21.
Which of the following should be most volatile?
I. CH3CH2CH2NH2
II. (CH3)3N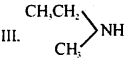
IV. CH3CH2CH3
(a) II
(b) IV
(c) I
(d) III
Answer
Answer: (b) IV
Question 22.
Identify the correct IUPAC name
(a) (CH3CH2)2NCH3 = N-Ethyl-N-methylethanamine
(b) (CH3)3CNH2 = 2-methylpropan-2-amine
(c) CH3NHCH (CH3)2 = N-Methylpropan-2-amine
(d) (CH3)2CHNH2 = 2, 2-Dimethyl-N-propanamine
Answer
Answer: (a) (CH3CH2)2NCH3 = N-Ethyl-N-methylethanamine
Question 23.
Which of the following from isocyanide on reaction with CHCl and KOH?
(a) C6H5NHCH3
(b) CH3C6H4NH2
(c) C6H5NHC4H9.
(d) C6H5N (C2H5)2
Answer
Answer: (b) CH3C6H4NH2
Question 24.
C3H8N cannot represent
(a) 1° ammine
(b) 2° ammine
(c) 3° ammine
(d) quartemary ammonium salt
Answer
Answer: (d) quartemary ammonium salt
Question 25.
Identify ‘Z’ in the sequence?![]()
(a) C6H5CN
(b) C6H5CONH2
(c) C6H5COOH
(d) C6H5CH2NH2
Answer
Answer: (c) C6H5COOH













0 Comments:
Post a Comment