CBSE Class 12 Chemistry – MCQ and Online Tests – Unit 10 – Haloalkanes and Haloarenes
Every year CBSE conducts board exams for 12th standard. These exams are very competitive to all the students. So our website provides online tests for all the 12th subjects. These tests are also very effective and useful for those who preparing for competitive exams like NEET, JEE, CA etc. It can boost their preparation level and confidence level by attempting these chapter wise online tests.
These online tests are based on latest CBSE Class 12 syllabus. While attempting these our students can identify the weak lessons and continuously practice those lessons for attaining high marks. It also helps to revise the NCERT textbooks thoroughly.
CBSE Class 12 Chemistry – MCQ and Online Tests – Unit 10 – Haloalkanes and Haloarenes
Question 1.
Criteria for purity of organic solid is
(a) boiling point
(b) melting point
(c) specific gravity
(d) none of these
Answer
Answer: (b) melting point
Question 2.
Hybridisation of carbon in ethane is
(a) sp3
(b) sp2
(c) sp
(d) sp3d2
Answer
Answer: (a) sp3
Question 3.
The negative part of the addendum (the molecule to be added) adds on the carbon atom of the double bond containing the least number of hydrogen atoms. This rule is known as
(a) Saytzeffs rule
(b) Peroxide rule
(c) Markovnikov’s rule
(d) van’t hoff rule
Answer
Answer: (c) Markovnikov’s rule
Question 4.
General formula of Alkene is
(a) CnH2n
(b) CnH2n+2
(c) CnH2n-2
(d) none of these
Answer
Answer: (a) CnH2n
Question 5.
Number of π bonds in ethyne is
(a) 1
(b) 2
(c) 3
(d) 4
Answer
Answer: (b) 2
Question 6.
Organic compound must contain an element
(a) oxygen
(b) carbon
(c) hydrogen
(d) nitrogen
Answer
Answer: (b) carbon
Question 7.
Which of the following is not correctly matched with its IUPAC name?
(a) CHF2CBrClF : 1-Bromo-1-chIoro-1, 2, 2-trifluoroethane
(b) (CCl3)3CCl : 2-(Trichloromethyl)-1, 1, 2, 3, 3-heptachloropropane
(c) CH3C (p-ClC6H4)2CH(Br)CH3 : 2-Bromo-3, 3-bis (4- chlorophenyl) butane
(d) o-BrC6H4CH (CH3) CH2CH3 : 2-Bromo-l- methylpropylbenzene
Answer
Answer: (b) (CCl3)3CCl : 2-(Trichloromethyl)-1, 1, 2, 3, 3-heptachloropropane
Question 8.
Which of the following compounds can yield only one monochlorinafed product upon free radical chlorination?
(a) 2, 2-Dimethylpropane
(b) 2-Methylpropane
(c) 2-Methylbutane
(d) n-Butane
Answer
Answer: (a) 2, 2-Dimethylpropane
Question 9.
Which of the following molecules has highest dipole moment?
(a) CH3Cl
(b) CH2Cl2
(c) CHCl5
(d) CCl4
Answer
Answer: (a) CH3Cl
Question 10.
The compound having general formula CnH2n+2 is
(a) Alkene
(b) Alkyne
(c) Alkane
(d) none of these
Answer
Answer: (c) Alkane
Question 11.
Halogen acids react with alcohols to form alkyl halides. The reaction follows a nucleophilic substitution mechanism. What will be the product of the following reaction?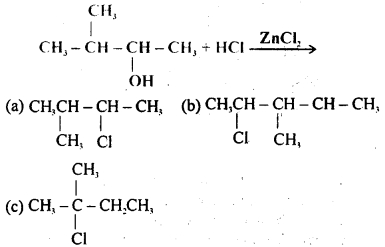
(d) CH3CH2CH2CH2CH2Cl
Answer
Answer: (a)
Question 12.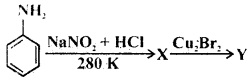
X and Y in the reaction are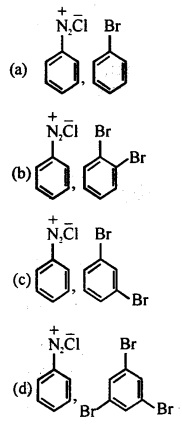
Answer
Answer: (a)
Question 13.
Good conductor of electricity and heat is
(a) Anthracite coke
(b) Diamond
(c) Graphite
(d) Charcoal
Answer
Answer: (c) Graphite.
Question 14.
Bromination of methane in presence of sunlight is a
(a) nucleophilic substitution
(b) free radical substitution
(c) electrophilic substitution
(d) nucleophilic addition
Answer
Answer: (b) free radical substitution
Question 15.
Which one of the following is not correct order of boiling .points of the alkyl/aryl halides?
(a) CHCl3 > CH2Cl2
(b) CH3(CH2)3CI > CH3(CH2)2Cl
(c) (CH3)3CCl > (CH3)2CHCH2Cl
(d) CH3(CH2)3Cl > CH3CH2CHClCH3
Answer
Answer: (c) (CH3)3CCl > (CH3)2CHCH2Cl
Question 16.
Which of the following reactions follows Markovnikov’s rule?
(a) C2H4 + HBr
(b) C3H6 + Cl6
(c) C3H6 + HBr
(d) C3H6 + Br2
Answer
Answer: (c) C3H6 + HBr
Question 17.
Which of the following compounds has the highest boiling point?
(a)CH3CH2CH2Cl
(b) CH3CH2CH2CH2Cl
(c) CH3CH(CH3)CH2Cl
(d) (CH3)3CCl
Answer
Answer: (b) CH3CH2CH2CH2Cl
Question 18.
Arrange the following compounds in-decreasing order of their boiling points
(i) CH3Br
(ii) CH3CH2Br
(iii) CH3CH2CH2Br
(iv) CH2CH2CH2CH2Br
(a) (i) > (ii) > (iii) > (iv)
(b) (iv) > (iii) > (ii) > (i)
(c) (i) > (iii) > (ii) > (iv)
(d) (iii) > (iv) > (i) > (ii)
Answer
Answer: (b) (iv) > (iii) > (ii) > (i)
Question 19.
Alkyl halides are immiscible in water though they are polar because
(a) they react with water to give alcohols
(b) they cannot form hydrogen bonds with water
(c) C -X bond cannot be broken easily
(d) they are stable compounds and are not reactive
Answer
Answer: (b) they cannot form hydrogen bonds with water
Question 20.
The reaction of toluene with chlorine in presence of FeCl3 gives predominantly.
(a) amixture of o-and p-chlorotoluene
(b) benzyl chloride
(c) m-chlorotuluene
(d) benzoyl chloride
Answer
Answer: (a) amixture of o-and p-chlorotoluene
Question 21.
Which of the following compounds will have highest melting point?
(a) Chlorobenzene
(b) o-Dichlorobenzene
(c) m-Dichlorobenzene
(d) p-Dichlorobenzene
Answer
Answer: (d) p-Dichlorobenzene
Question 22.
Ethyl alcohol is obtained when ethyl chloride is boiled with
(a) alcoholic KOH
(b) aqueous KOH
(c) water
(d) aqueous KMnO4
Answer
Answer: (b) aqueous KOH
Question 23.
The hybridisation of carbon in diamond is
(a) sp3
(b) sp2
(c) sp
(d) dsp2
Answer
Answer: (a) sp3
Question 24.
Which of the following alkyl halides undergoes hydrolysis with aqueous KOH at the fastest rate?
(a) CH3CH2CH2Cl
(b) CH3CH2Cl
(C) CH3CH2CH2CH2Cl
(d) CH3CH2CH (Br) CH3
Answer
Answer: (d) CH3CH2CH (Br) CH3
Question 25.
The reaction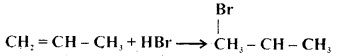
is an example of
(a) nucleophilic addition
(b) free radical addition
(c) electrophilic addition
(d) electrophilic substitution
Answer
Answer: (c) electrophilic addition
Question 26.
Alkene gives which of the following reactions?
(a) Addition reaction
(b) Substitution reaction
(c) Both (a) and (b)
(d) None of these
Answer
Answer: (c) Both (a) and (b)
Question 27.
Butane nitrile can be prepard by heating.
(a) propyl alcohol with KCN
(b) butyl chloride with KCN
(c) butyl alcohol with KCN
(d) propyl chloride with KCN
Answer
Answer: (d) propyl chloride with KCN
Question 28.
Which of the following reactions will give the major and minor products?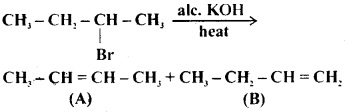
(a) (a) is major product and (b) is minor product
(b) (a) is minor product and (b) is major product
(c) Both (a) and (b) are major products
(d) Only (b) is formed and (a) is not formed
Answer
Answer: (a) (a) is major product and (b) is minor product
Question 29.
Which of the following is the most reactive towards nucleophilic substitution reaction?
(a) ClCH2– CH = CH2
(b) CH2 = CH-Cl
(c) CH3CH = CH-Cl
(d) C6H6Cl
Answer
Answer: (a) ClCH2-CH = CH2
Question 30.![]()
The final product in the reaction is
(a) CH3OH
(b) HCOOH
(c) CH3OH
(d) CH3COOH
Answer
Answer: (d) CH3COOH
Question 31.
The end product (Q) is in the following sequence of reaction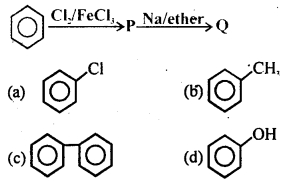
Answer
Answer: (c)
Question 32.
Methyl bromide reacts with AgF to give methyl fluoride and silver bromide. This reaction is called
(a) Fittig reaction
(b) Swarts reaction
(c) Wurtz reaction
(d) Finkelstein reaction
Answer
Answer: (b) Swarts reaction
Question 33.
The alkyl halide is converted into an alcohol by
(a) elimination
(b) dehydrohalogenation
(c) addition
(d) substitution
Answer
Answer: (d) substitution
Question 34.
A mixture of 1-chloropropane and 2-chloropropane when treated with alcoholic KOH gives
(a) prop-1-ene
(b) prop-2-ene
(c) a mixture of prop-1 -ene and prop-2-ene
(d) propanol
Answer
Answer: (a) prop-1-ene
Question 35.
Valency of carbon is
(a) 1
(b) 2
(c) 3
(d) 4
Answer
Answer: (d) 4
Question 36.
In which of the following allotropes of carbon, percentage of carbon is maximum?
(a) Wood charcoal
(b) Coconut charcoal
(c) Graphite
(d) None of these
Answer
Answer: (c) Graphite
Question 37.
In SN2 reactions with the sequence of bond breaking and bond formation is as follows
(a) bond breaking is followed by formation
(b) bond formation is followed by breaking
(c) bond breaking and formation are simultaneously
(d) bond breaking and formation take place randomly
Answer
Answer: (c) bond breaking and formation are simultaneously
Question 38.
Single bond length between carbon-carbon is
(a) 1.34 Å
(b) 1.20 Å
(c) 1.54 Å
(d) none of these
Answer
Answer: (c) 1.54 Å
Question 39.
Grignard reagents are formed by the reaction of alkyl halides by warming
(a) with alcoholic solution
(b) with MgCl2
(c) Mg in presence of dry ether
(d) with MgCO3
Answer
Answer: (c) Mg in presence of dry ether
Question 40.
An alkyl halide, RX reacts with KCN to given propane nitrile, RX is
(a) C3H7Br
(b) C4H9Br
(c) C2H5Br
(d) C5H11Br
Answer
Answer: (c) C2H5Br













0 Comments:
Post a Comment