CBSE Class 12 Chemistry – MCQ and Online Tests – Unit 16 – Chemistry in Everyday Life
Every year CBSE conducts board exams for 12th standard. These exams are very competitive to all the students. So our website provides online tests for all the 12th subjects. These tests are also very effective and useful for those who preparing for competitive exams like NEET, JEE, CA etc. It can boost their preparation level and confidence level by attempting these chapter wise online tests.
These online tests are based on latest CBSE Class 12 syllabus. While attempting these our students can identify the weak lessons and continuously practice those lessons for attaining high marks. It also helps to revise the NCERT textbooks thoroughly.
CBSE Class 12 Chemistry – MCQ and Online Tests – Unit 16 – Chemistry in Everyday Life
Question 1.
The drugs which are given to the patients suffering from anxiety and metnal tension are known as
(a) tranquilizers
(b) analgesics
(c) antimicrobials
(d) antibiotics
Answer
Answer: (a) tranquilizers
Question 2.
Antimicrobial drugs include
(i) antiseptics
(ii) antibiotics
(iii) disinfectants
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i), (ii) and (iii)
Answer
Answer: (d) (i), (ii) and (iii)
Question 3.
Which of the following can be used as an analgesic without causing addiction?
(a) Morphine
(b) Aspirin
(c) Heroin
(d) Codeine
Answer
Answer: (b) Aspirin
Question 4.
Which of the following will not act as antacid?
(a) Sodium hydrogencarbonate
(b) Magnesium hydroxide
(c) Sodium carbonate
(d) Aluminium hydroxide
Answer
Answer: (c) Sodium carbonate
Question 5.
Which of the following will not act as a tranquilizer?
(a) Equanil
(b) Analgin
(c) Meprobamate
(d) Chlordiazepoxide
Answer
Answer: (b) Analgin
Question 6.
Antihistamines are not helpful
(a) in curing nasal allergies
(b) in treating rashes caused by itching
(c) in bringing down acute fever
(d) in vasodilation
Answer
Answer: (c) in bringing down acute fever
Question 7.
The chemical substances used to bring down body temperature in high fever are known as
(a) analgesics
(b) antipyretics
(c) antihistamines
(d) tranquilizers
Answer
Answer: (b) antipyretics
Question 8.
Drugs that bind to the receptor site and inhibit its natural function are called
(a) agonistic drugs
(b) antagonists drugs
(c) antimicrobial drugs
(d) allosteric drugs
Answer
Answer: (b) antagonists drugs
Question 9.
A drug which acts as antipyretic as analgesic is
(a) chloroquin
(b) penicillin
(c) chlorodiazeposide
(d) 4-acetamidophenol
Answer
Answer: (d) 4-acetamidophenol
Question 10.
Terfenadine is commonly used as
(a) antihistamine
(b) antibiotic
(c) antimicrobial
(d) antifertility drug
Answer
Answer: (a) antihistamine
Question 11.
Barbituric acid and its derivatives are well known as
(a) tranquilizers
(b) antiseptics
(c) analgesics
(d) antipyretics
Answer
Answer: (a) tranquilizers
Question 12.
The main cause of acidity in the stomach is
(a) release of extra gastric acids which decrease the pH level
(b) indigestion and pain in large intestine
(c) increase the pH level in the stomach
(d) release of extra bile juice which increases alkaline medium in stomach
Answer
Answer: (a) release of extra gastric acids which decrease the pH level
Question 13.
Which of the following statements is not correct about penicillin?
(a) Penicillin G has a narrow spectrum
(b) It is extracted from antibacterial fungus Penicillium
(c) Ampicillin and Amoxycilin are synthetic modification of penicillins
(d) It has bacteriostatic effect
Answer
Answer: (d) It has bacteriostatic effect
Question 14.
Which of the following statements is not correct?
(a) Some disinfectants can be used as antiseptic at low concehtration
(b) Aspirin is analgesic and natipyretic
(c) Norethindrone is an antihistamines
(d) Chloramphenicol is a broad spectrum antibiotic
Answer
Answer: (c) Norethindrone is an antihistamines
Question 15.
An ester which is used as a medicine
(a) ethyl acetate
(b) methyl acetate
(c) methyl salicylate
(d) ethyl benzoate
Answer
Answer: (c) methyl salicylate
Question 16.
Barbiturates acts as
(a) hypnotic i.e., sleep producing agents
(b) non-narcotic analesics
(c) activator of neurotransmitters
(d) antilallergic drugs
Answer
Answer: (a) hypnotic i.e. sleep producing agents
Question 17.
Which of the following is a narcotic analgesic?
(a)Ibuprofen
(b) Aspirin
(c) Paracetamol
(d) Morphine
Answer
Answer: (d) Morphine
Question 18.
The structure given below is known as
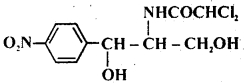
(a) pronstosil
(b) sulphapyridine
(c) chloramphenicol
(d) chloroxylenol
Answer
Answer: (c) chloramphenicol
Question 19.
Which ofthe following is not an antibiotic?
(a) Chloramphenicol
(b) Ofloxacin
(c) Penicillin
(d) Prontosil
Answer
Answer: (d) Prontosil
Question 20.
The term ‘broad spectrum antibiotics’ means
(a) bacterial antibiotics
(b) bacteriostatic antibiotics
(c) which kill or inhibit a wide range of gram -ve and gram +ve bacteria
(d) which kill or inhibit all types of gram +ve bacteria
Answer
Answer: (c) which kill or inhibit a wide range of gram -ve and gram +ve bacteria
Question 21.
A drug which is effective in curring malaria is
(a) aspirin
(b) quinine
(c) morphine
(d) analgin
Answer
Answer: (b) quinine
Question 22.
Which of the following statements is incorrect?
(a) Aspirin is both analgesic and antipyretic
(b) Ampicillin is a natural antibiotic
(c) Salvarsan is toxic to human beings
(d) Some disinfectants are used as antiseptics in lower concentrations
Answer
Answer: (b) Ampicillin is a natural antibiotic
Question 23.
Which of the following antibiotic is bactericidal?
(a) Erythromycin
(b) Tetracycline
(c) Penicillin
(d) Chloramphenicol
Answer
Answer: (c) Penicillin
Question 24.
The main constituents of dettol are
(a) chloramphenicol + glycerol
(b) 2-3% solution of iodine in alcohol
(c) 0.2% solution of phenol
(d) chloroxylenol and terpineol
Answer
Answer: (d) chloroxylenol and terpineol
Question 25.
The use of chemicals for therapeutic effect is called
(a) chemotherapy
(b) physiotherapy
(c) angiotherapy
(d) polytherapy
Answer
Answer: (a) chemotherapy
Question 26.
Which of the following is not an antidepressants?
(a) Ipronizaid
(b) Phenelzine
(c) Equanil
(d) Salvarsan
Answer
Answer: (d) Salvarsan
Question 27.
The antibiotic which is effective against certain strain of cancer cells
(a) dysidazirine
(b) sulphanilamide
(c) vancomycin
(d) ofloxacin
Answer
Answer: (a) dysidazirine
Question 28.
Which among the following is not an antibiotic?
(a) Penicillin
(b) Oxytocin
(c) Erythromycin
(d) Tetracyclin
Answer
Answer: (b) Oxytocin
Question 29.
The use of aspartame is limited to cold foods and drinks because
(a) it is unstable to heat and decomposes at cooking temperature
(b) it is 500 times sweeter than cane sugar
(c) it becomes bitter at cooking temperature
(d) it reacts with the food at cooking temperature
Answer
Answer: (a) it is unstable to heat and decomposes at cooking temperature
Question 30.
Which one is a broad spectrum antibiotic?
(a) Chloramphenicol
(b) Plasmoquin
(c) Xylocaine
(d) Antiseptic
Answer
Answer: (a) Chloramphenicol
Question 31.
Some drugs do not bind to the enzyme’s artive site, instead bind to a different site of enzyme. This site is called
(a) allosteric site
(b) substrate site
(c) ionic site
(d) competitive site
Answer
Answer: (a) allosteric site
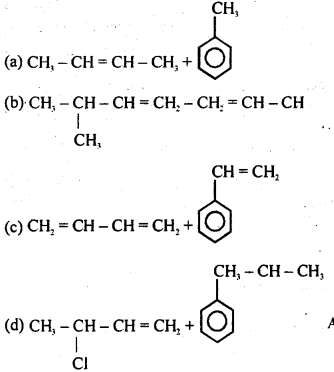
Question 32.
Which of the following compounds represents an analgesic?
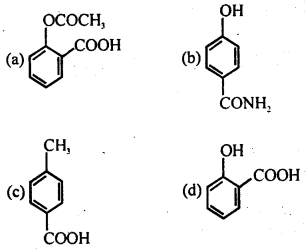
Answer
Answer: (a)
Question 33.
Name an artificial sweetner which is derivative of sucrose
(a) Saccharine
(b) Sucrolose
(c) Sucrobenzamide
(d) Aspartame
Answer
Answer: (b) Sucrolose
Question 34.
What is tincture of iodine?
(a) 2-3% solution of iodine in alcohol-water mixture
(b) A mixture of iodine in chloroxylenol
(c) A mixture of 0.2% phenol and 2-3% iodine in water
(d) 2-3% solution of iodine in potassium iodide
Answer
Answer: (a) 2-3% solution of iodine in alcohol-water mixture
Question 35.
What is the problem faced while using alitame as artificial sweetener?
(a) It decomposes when added to the food items
(b) It provides a huge number of calories to the food
(c) It is difficult to control the sweetness of food while using it
(d) It increases the volume of the contents to a large extent
Answer
Answer: (c) It is difficult to control the sweetness of food while using it