CBSE Class 11 Chemistry – MCQ and Online Tests – Unit 6 – Thermodynamics
Every year CBSE schools conducts Annual Assessment exams for 6,7,8,9,11th standards. These exams are very competitive to all the students. So our website provides online tests for all the 6,7,8,9,11th standard’s subjects. These tests are also very effective and useful for those who preparing for any competitive exams like Olympiad etc. It can boost their preparation level and confidence level by attempting these chapter wise online tests.
These online tests are based on latest CBSE syllabus. While attempting these our students can identify the weak lessons and continuously practice those lessons for attaining high marks. It also helps to revise the NCERT textbooks thoroughly.
CBSE Class 11 Chemistry – MCQ and Online Tests – Unit 6 – Thermodynamics
Question 1.
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283.0 kJ mol-1 respectively. The enthalpy of formation of carbon monoxide is:
(a) -676 kJ
(b) 110.5 kJ
(c) -110.5 kJ
(d) 676.5 kJ
Answer
Answer: (c) -110.5 kJ
Explanation:
C(s) + O2 à CO2 ?H1 = -393.5
CO + (\(\frac {1}{2}\)) O2 à CO2 ?H2 = -283.0
C(s) + (\(\frac {1}{2}\)) O2 à CO ?H = ?H1 – ?H2
= -393.5 + 283 = -110.5 KJ
Question 2.
The amount of the heat released when 20 ml 0.5 M NaOH is mixed with 100 ml 0.1 M HCl is x kJ. The heat of neutralization is
(a) -100 × kJ/mol
(b) -50 × kJ/mol
(c) 100 × KJ/mol
(d) 50 × kJ/mol
Answer
Answer: (a) -100 × kJ/mol
Explanation:
Normality of NaOH = Molarity × acidity
= 0.5 × 1 = 0.5 N
Total heat q produced = x kJ
Heat of neutralisation
= [(q)/ (Volume of acid or base)] ×1000× (1/normality of acid or base)
= (\(\frac {x}{20}\)) × 1000 × (\(\frac {1}{0.5}\))
= 100 x kJmol-1
Since heat is liberated, heat of neutralisation = -100 x kJmol-1
Question 3.
The enthalpy of vaporisation of a substance is 8400 J mol-1 and its boiling point is –173°C. The entropy change for vaporisation is :
(a) 84 J mol-1K-1
(b) 21 J mol-1K-1
(c) 49 J mol-1K-1
(d) 12 J mol-1K-1
Answer
Answer: (a) 84 J mol-1K-1
Explanation:
?S = (qrev)/ (T)
= (\(\frac {8400}{100}\))
= 84 J mol-1K-1
Question 4.
The species which by definition has ZERO standard molar enthalpy of formation at 298 K is
(a) Br2(g)
(b) Cl2(g)
(c) H2O(g)
(d) CH4(g)
Answer
Answer: (b) Cl2(g)
Explanation:
This is possible only for elements, chlorine is a gas at this temperature, but bromine is a liquid, so it is possible only for chlorine.
Question 5.
In a reversible process the system absorbs 600 kJ heat and performs 250 kJ work on the surroundings. What is the increase in the internal energy of the system?
(a) 850 kJ
(b) 600 kJ
(c) 350 kJ
(d) 250 kJ
Answer
Answer: (c) 350 kJ
Explanation:
?E = q + w
= (600 – 250)
?E = 350 J
Question 6.
Which of the following is true for the reaction? H2O (l) ? H2O (g) at 100° C and 1 atm pressure
(a) ?S = 0
(b) ?H = T ?S
(c) ?H = ?U
(d) ?H = 0
Answer
Answer: (a) ?S = 0
Explanation:
Equilibrium
Therefore, ? G = 0 = ? H – T ? S
Or T? S = ?H
Question 7.
Calculate the heat required to make 6.4 Kg CaC2 from CaO(s) and C(s) from the reaction: CaO(s) + 3 C(s) ? CaC2(s) + CO (g) given that ?f Ho (CaC2) = -14.2 kcal. ?f H° (CO) = -26.4 kcal.
(a) 5624 kca
(b) 1.11 × 104 kcal
(c) 86.24 × 10³
(d) 1100 kcal
Answer
Answer: (b) 1.11 × 104 kcal
Explanation:
n = (Mass)/ (Molecular weight)
= (6.4 × 10³)/ (64)
= 100
For 1 mole of CaC2
? H = ?Hf (CaC) + Hf (CO) – Hf (CaO)
= -14.2 – 26.4 + 151.6 = 111.1 kcal
For 100 moles, ?H = 1.11 × 104 Kcal
Question 8.
In a system where ?E = -51.0 kJ, a piston expanded against a pext of 1.2 atm giving a change in volume of 32.0 L. What was the change in heat of this system?
(a) -36 kJ
(b) -13 kJ
(c) -47 kJ
(d) 24 kJ
Answer
Answer: (c) -47 kJ
Explanation:
w = -1.2 (32) × 101.3
= – 3.89 KJ
= -4 (approx.)
= ? E = – 51.0 KJ
Therefore, E = q + w
– 51 = q – 4
Therefore, q = – 47 KJ
Question 9.
A system absorb 10 kJ of heat at constant volume and its temperature rises from 270 C to 370 C. The value of ? U is
(a) 100 kJ
(b) 10 kJ
(c) 0 kJ
(d) 1 kJ
Answer
Answer: (b) 10 kJ
Explanation:
At constant volume w = 0
Therefore, ? U = q = 10 kJ
Question 10.
The bond energy (in kcal mol-1) of a C-C single bond is approximately
(a) 1
(b) 10
(c) 83-85
(d) 1000
Answer
Answer: (c) 83-85
Explanation:
C–C bond 83–85 kcal/mol
It is the energy required to break the bond .It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K (absolute zero)
Question 11.
Third law of thermodynamics provides a method to evaluate which property?
(a) Absolute Energy
(b) Absolute Enthalpy
(c) Absolute Entropy
(d) Absolute Free Energy
Answer
Answer: (c) Absolute Entropy
Explanation:
The Third Law of Thermodynamics is concerned with the limiting behavior of systems as the temperature approaches absolute zero. Most thermodynamics calculations use only entropy differences, so the zero point of the entropy scale is often not important. However, the Third Law tells us about the completeness as it describes the condition of zero entropy.
Question 12.
One mole of which of the following has the highest entropy?
(a) Liquid Nitrogen
(b) Hydrogen Gas
(c) Mercury
(d) Diamond
Answer
Answer: (b) Hydrogen Gas
Explanation:
The measure of randomness of a substance is called entropy. Greater the randomness of molecules of a substance greater is the entropy. Here hydrogen gas has more entropy as it shows more randomness/disorderliness due to less molar mass than all the given substances and also in the gas phase.
Question 13.
Which thermodynamic function accounts automatically for enthalpy and entropy both?
(a) Helmholtz Free Energy (A)
(b) Internal Energy (E)
(c) Work Function
(d) Gibbs Free Energy
Answer
Answer: (d) Gibbs Free Energy
Explanation:
Gibbs free energy combines the effect of both enthalpy and entropy. The change in free energy (?G) is equal to the sum of the change of enthalpy (?H) minus the product of the temperature and the change of entropy (?S) of the system.
?G = ?H – T?S
?G predicts the direction in which a chemical reaction will go under two conditions: (1) constant temperature and (2) constant pressure.
If ?G is positive, then the reaction is not spontaneous (it requires the input of external energy to occur) and if it is negative, then it is spontaneous (occurs without the input of any external energy).
Question 14.
Which of the following salts will have maximum cooling effect when 0.5 mole of the salt is dissolved in same amount of water. Integral heat of solution at 298 K is given for each salt?
(a) KNO3 (?H = 35.4 kJ mol-1)
(b) NaCl (?H = 5.35 kJ mol-1)
(c) HBr (?H = -83.3 kJ mol-1)
(d) KOH ( ?H = -55.6 kJ mol-1)
Answer
Answer: (a) KNO3 (?H = 35.4 kJ mol-1)
Explanation:
More the heat absorbed, more will be the cooling effect. Hence, more the positive value of ? H, more the cooling effect.
Question 15.
Standard enthalpy of vapourisation ?Hvap for water at 100oC is 40.66 kJmol-1. The internal energy of vapourisation of water at 100°C (in kJmol-1) is (Assume water vapour to behave like an ideal gas)
(a) 43.76
(b) 40.66
(c) 37.56
(d) -43.76
Answer
Answer: (c) 37.56
Explanation:
For gaseous reactants and products, we have a relation between standard enthalpy of vaporization (?Hvap) and standard internal energy (?E) as-
?Hvap = ?E+ ?ng? RT whereas,
?ng = n2? – n1, i.e., difference between no. of moles of reactant and product.
For vaporization of water,
H2O (l) ? H2O(g)
?Therefore, ?ng = 1 – 0 = 1
Therefore, ?Hvap = ?E+ ?ng RT
? ?E = ?Hvap – ?ng
?RT = 40.63 – (1×8.314×10-3 × 373) = 37.53 KJ/mol
Hence the value ?E for this process will be 37.53KJ/mol.
Question 16.
Based on the first law of thermodynamics, which one of the following is correct?
(a) For an isothermal process, q = +w
(b) For an isochoric process, ?U = -q
(c) For an adiabatic process, ?U = -w
(d) For a cyclic process, q = -w
Answer
Answer: (d) For a cyclic process, q = -w
Explanation:
(1) ?U = q + w. For an isochoric process, w = -P?V = 0. Hence, ?U = qv
(2) For an adiabatic process, q = 0. Hence, ?U = w
(3 ) For an isothermal process, ?U = 0 Hence, q = -w
(4) For a cyclic process , ?U = 0. Hence, q = -w
Question 17.
A system absorb 10 kJ of heat at constant volume and its temperature rises from 270°C to 370°C. The value of ? U is
(a) 100 kJ
(b) 10 kJ
(c) 0 kJ
(d) 1 kJ
Answer
Answer: (b) 10 kJ
Explanation:
At constant volume w = 0
Therefore, ? U = q = 10 kJ
Question 18.
The temperature of the system decreases in an ______.
(a) Adiabatic Compression
(b) Isothermal Expansion
(c) Isothermal Compression
(d) Adiabatic Expansion
Answer
Answer: (d) Adiabatic Expansion
Explanation:
In adiabatic process heat is neither added nor removed from system. So the work done by the system (expansion) in adiabatic process will result in decrease of internal energy of that system (from first law).
As internal energy is directly proportional to the change in temperature there will be temperature drop in an adiabatic process.
Question 19.
An ideal gas is taken around the cycle ABCA as shown in P-V diagram The next work done by the gas during the cycle is equal to: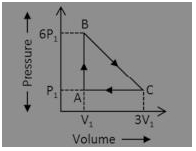
(a) 12P1V1
(b) 6P1V1
(c) 5P1V1
(d) P1V1
Answer
Answer: (c) 5P1V1
Explanation:
Work done = Area under P-V graph = (\(\frac {1}{2}\)) (5P1) (2V1) = 5P1 V1
Question 20.
In which of the following process, a maximum increase in entropy is observed?
(a) Dissolution of Salt in Water
(b) Condensation of Water
(c) Sublimation of Naphthalene
(d) Melting of Ice
Answer
Answer: (c) Sublimation of Naphthalene
Explanation:
The order of entropy in solid, liquid and gas is gas > liquid > solid .Hence, in sublimation of naphthalene, maximum increase in entropy is observed.














0 Comments:
Post a Comment